最新论文
Na L, Tang YD, Wang C, Liu C, Wang X. Rhesus monkey TRIM5alpha SPRY domain contributes to AP-1 activation. J Biol Chem. 2017 Dec 1. pii: jbc.RA117.000127.doi: 10.1074/jbc.RA117.000127.
发布日期:2017-12-12 14:33
浏览次数:
Rhesus monkey TRIM5alpha SPRY domain contributes to AP-1 activation.
Na L1, Tang YD1, Wang C1, Liu C1, Wang X2.
J Biol Chem.
Abstract
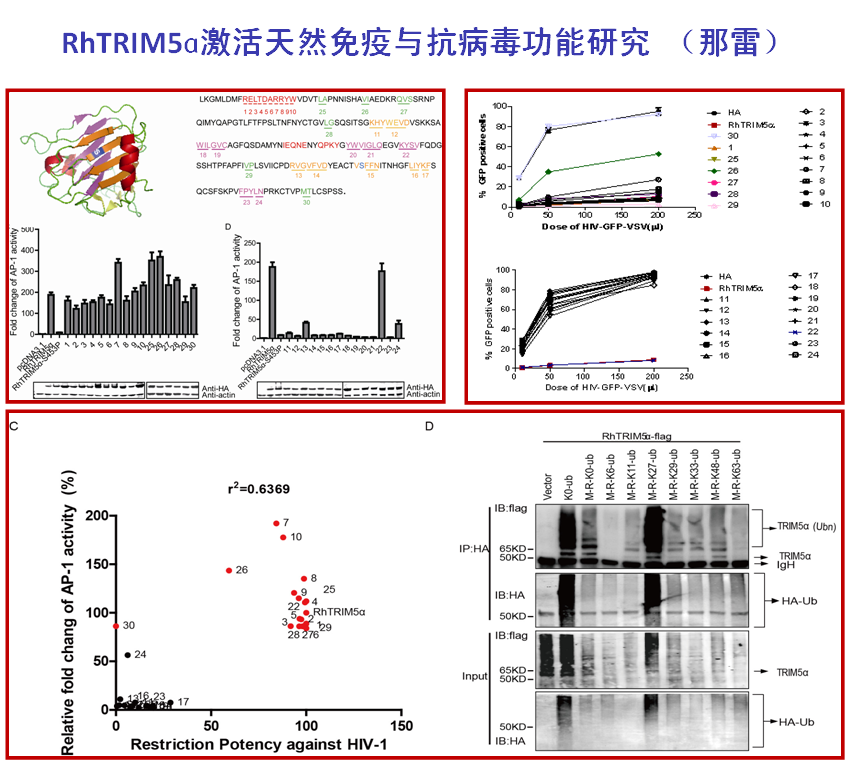
TRIM5α is an important host restriction factor which could potently block retrovirus infection. The SPRY domain of TRIM5α mediates post-entry restriction by recognition of and binding to the retroviral capsid. Human TRIM5α also functions as an innate immune sensor to activate AP-1 and NF-κB signaling, which subsequently restrict virus replication. Previous studies have shown that the AP-1 and NF-κB signaling activation relies on the RING motif of TRIM5α. In this study, we have demonstrated that the SPRY domain is essential for rhesus macaca TRIM5α to activate AP-1, but not NF-κB signaling. The AP-1 activation mainly depends on all the β-sheet barrel on SPRY structure of TRIM5α. Furthermore, the SPRY mediated auto-ubiquitination of TRIM5α is required for AP-1 activation. This study reports that rhesus macaca TRIM5α mainly undergoes Lys27-linked and Met1-linked auto-polyubiquitination. Finally, we found that the TRIM5α signaling function was positively correlated with its retroviral restriction activity. This study has discovered an important role of the SPRY domain in immune signaling and antiviral activity and further expanded our knowledge of the antiviral mechanism of TRIM5α.